The pharmaceutical industry initially had no interest in the drug
html[data-range=”xlarge”] figure image img.img-006aff1b66692b9f5bcb8b6bad997ec6ul31fejw { width: 774px; height: 435px; }HTML[data-range=”large”] figure image img.img-006aff1b66692b9f5bcb8b6bad997ec6ul31fejw { width: 548px; height: 308px; }HTML[data-range=”small”] image figure img.img-006aff1b66692b9f5bcb8b6bad997ec6ul31fejw, html[data-range=”medium”] figure image img.img-006aff1b66692b9f5bcb8b6bad997ec6ul31fejw { width: 564px; height: 317px; }HTML[data-range=”small”] .article__image-embed, html[data-range=”medium”] .article__image-embed { width: 564px; margin: auto 0 30px; }
It all began in Cheshire, England in 1962. A company called Imperial Chemical Industries (ICI) was testing a compound that promised to become a birth control pill.
The substance known as ICI46474, which had been synthesized by a female chemist Dora Richardson, actually had contraceptive effects on rats.
Potentially, it could become a morning-after pill, which can be taken within hours of intercourse to prevent pregnancy.
But, almost a decade later, after several tests, it was discovered that in humans the molecule had the opposite effect: it increased fertility by stimulating ovulation.
Research with ICI46474 would have been abandoned altogether had it not been for the intervention of the head of the team of scientists, Arthur Walpole, who threatened to resign if the project did not go ahead. In parallel with the study of the compound as a contraceptive, research has been conducted with it as a treatment for breast cancer.
In 1971, the first clinical trial of ICI46474 was carried out at Christie’s Hospital in Manchester and produced positive results, with “the particular advantage of a low incidence of troublesome side effects”.
However, this was not enough for the pharmaceutical company. According to accounts by Dora Richardson, Walpole and her colleagues were told they should have been looking for a birth control pill and not an anticancer agent.
Despite growing clinical evidence of the compound’s utility in this field, the market for an anticancer drug was considered small, in part because of the poor prognosis associated with the disease.
Sales estimates produced by Imperial Chemical Industries’ marketing department indicated that sales would not cover research and development costs and would not generate an adequate return for the company.
“It’s all about the money in the history of the pharmaceutical industry,” Professor V. Craig Jordan, who would later help publicize the drug, told BBC Witness.
However, by the time the company decided to end the program, the breast cancer tests had already generated several publications, sparking worldwide interest in the compound. Under pressure, the drugmaker reversed its decision, Walpole stayed on, and the project was saved.
In February 1973 the company applied for product registration under Health Surveillance and Nolvadex was launched in the UK for anovulatory infertility and the palliative treatment of breast cancer.
From failure to success
ICI46474 is designed to act as an anti-estrogen remedy.
In 1896, George Beatson, a pioneer of cancer surgery, discovered that he could prolong the lives of women with breast cancer by surgically removing their ovaries, a major source of estrogen.
This gave the researchers the first clue that these female hormones were involved in the growth and development of breast cancer.
Over the next fifty years, doctors experimented with a variety of products to inhibit estrogen and treat breast cancer.
While their efforts were sometimes successful, the side effects were too severe for widespread use.
In the mid-1960s, when ICI46474 was being tested, despite the growing awareness of breast cancer, the research seemed to have come to a standstill.
A mastectomy, in which the cancerous area of a woman’s breast is removed, along with chemotherapy or radiation therapy, used to be considered the only way forward.
This would soon change.
Thanks to a combination of luck, common sense, and a “war on cancer” that began in the United States in the 1970s, there has been renewed interest in developing an estrogen blocker to treat breast cancer.
But in the US, the most coveted market by pharmaceutical companies, ICI46474, which came to be called tamoxifen, took a while to get approved. Walpole died in 1977, without seeing his research progress and medicine become a worldwide success.
In 1977, “there was a breakthrough when Dora Richardson gave me tamoxifen metabolites,” says Craig Jordan. A metabolite is a byproduct of what happens when the body breaks down the drug and turns it into other substances;
“My lab team showed that a tamoxifen metabolite was 100 times more potent than tamoxifen as an anti-estrogen. It was a drug like no other that had ever been seen, it was that strong,” says Jordan.
“When I brought the information to ICI, the room fell silent. They asked me to respect not to say anything about what I had discovered. I agreed because I wanted tamoxifen to be successful in the United States.”
While ICI has received the packet of metabolites, Jordan has not released his finding. Then he published an article announcing the discovery of the most powerful anti-estrogen in the world, which became the basis for all subsequent anti-estrogens.
Tamoxifen was approved for use in the United States in late 1977, an important milestone in the drug’s path to global success.
This is how a chemical compound that failed to live up to its initial promise became a wonder drug that was included in the World Health Organization’s (WHO) List of Essential Medicines.
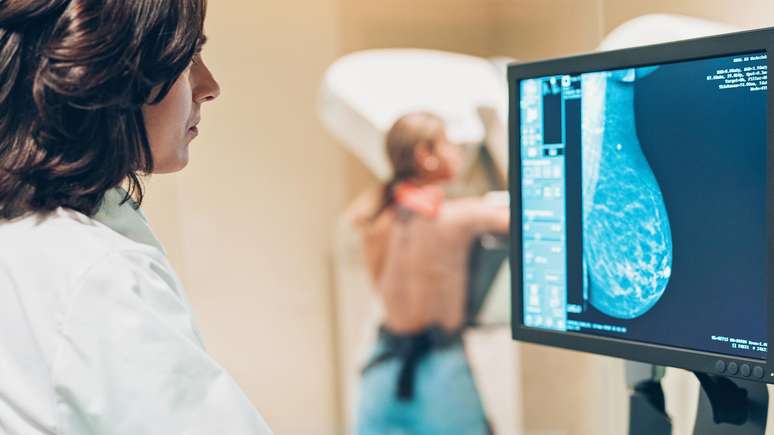
How tamoxifen works
Breast cancers that have estrogen receptors are called estrogen receptor positive (or ER+) cancers.
This means that the tumors feed on this female hormone, which circulates in the woman’s bloodstream.
Being estrogen dependent is the Achilles heel of ER+ tumors: It makes them sensitive to drugs like tamoxifen, which prevent estrogen from affecting cancer cells.
The drug works as if it were blocking a door: it binds to the estrogen receptor, preventing the hormone from entering and thus stopping the tumor from advancing.
That’s why it’s considered one of the greatest advances in cancer treatment.
According to WHO, breast cancer is the most commonly diagnosed type of cancer, accounting for 1 in 8 cancer diagnoses worldwide. In 2020 alone, there were an estimated 2.3 million new cases of breast cancer worldwide and an estimated 685,000 deaths from the disease.
And about 70% of breast cancers are ER+.
Source: Terra
Ben Stock is a lifestyle journalist and author at Gossipify. He writes about topics such as health, wellness, travel, food and home decor. He provides practical advice and inspiration to improve well-being, keeps readers up to date with latest lifestyle news and trends, known for his engaging writing style, in-depth analysis and unique perspectives.








