The pharmaceutical company Pfizer has reported that it intends to submit a request for approval of the immunizer to Anvisa in the coming weeks
the pharmacist pfizer has announced that it intends to submit to the National Health Surveillance Agency (Anvis) in the coming weeks, an application for approval of a new vaccine against respiratory syncytial virus (RSV), the leading cause of bronchiolitis. The vaccine is intended for pregnant women (with the aim of protecting newborns who inherit the mother’s antibodies) and also for the elderly. The vaccine has already been approved in the United States and the European Union.
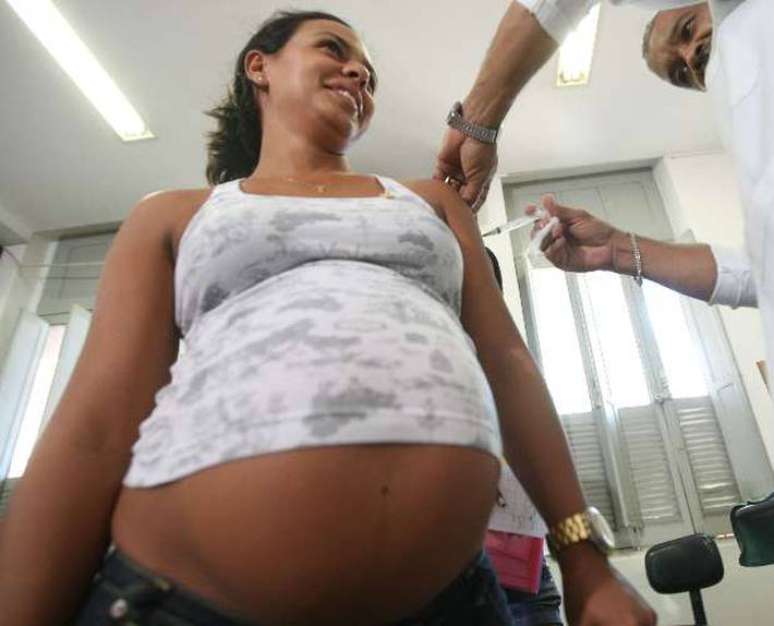
The Abysvo immunizer is indicated for pregnant women 32 to 36 weeks of gestation to elicit an immune reaction against lower respiratory tract infections caused by RSV in infants and toddlers up to six months of age – the main risk group for severe forms of virus infection. Seniors over 60 are also more vulnerable to developing serious respiratory diseases.
Bronchiolitis is acute inflammation of the terminal pulmonary bronchioles; that is, the finer branches that serve to conduct the air into the lungs. RSV is the main culprit in cases of bronchiolitis and pneumonia in children and the elderly.
html[data-range=”xlarge”] figure image img.img-d2143174558e84f98d2bd6e7f0518bf3ntnbsw6i { width: 774px; height: 626px; }HTML[data-range=”large”] figure image img.img-d2143174558e84f98d2bd6e7f0518bf3ntnbsw6i { width: 548px; height: 443px; }HTML[data-range=”small”] figure image img.img-d2143174558e84f98d2bd6e7f0518bf3ntnbsw6i, html[data-range=”medium”] figure image img.img-d2143174558e84f98d2bd6e7f0518bf3ntnbsw6i { width: 564px; height: 456px; }HTML[data-range=”small”] .article__image-embed, html[data-range=”medium”] .article__image-embed {width: 564px; margin: auto 0 30px; }
The disease is the leading cause of hospitalization of children up to four years of age in Brazil. The virus has a higher incidence in the colder months, autumn and winter, but circulates throughout the year. RSV is transmitted through the air via saliva particles.
Anvisa’s request for approval is based on the results of international clinical studies which demonstrated the safety and efficacy of the vaccine in protecting newborns and the elderly. The phase 3 study involved the participation of more than seven thousand pregnant women in 18 research centers around the world, four of them in Brazil.
The study showed that the vaccine was able to prevent the onset of serious respiratory diseases in 82% of children up to three months and in 69% of those up to six months. In the elderly, protection against serious pathologies reaches 85.7%.
“This is the first and only immunization available to protect infants immediately after birth and up to six months of age against RSV,” said Pfizer Chief Medical Officer in Brazil, Adriana Ribeiro.
“The virus is the main cause of bronchiolitis, an infection that can have serious consequences especially for children under the age of six months,” said Pfizer’s medical director in Brazil, Adriana Ribeiro.
There is currently no vaccine in Brazil to prevent RSV. Another vaccine, from GSK, has already been approved abroad, but only for the elderly.
The Brazilian Society of Pediatrics and the Brazilian Society of Vaccinations recommend the use of the drug palivizumab (five injections are planned in the months of greatest circulation of the virus) for infants and children. The government, however, only offers the drug free to premature babies.
The latest edition of Fiocruz’s InfoGripe epidemiological bulletin, published last Thursday, 24, shows an increase in new cases of severe acute respiratory syndrome in Espírito Santo, Roraima and São Paulo in children aged 2 to 14 years. One fifth of these cases are caused by RSV.
“The search for this vaccine is old, it’s not a passing fad; the problem is that until now no one has been able to make something that works,” explained pediatrician and infectious disease Ana Paula Burian, president of the Brazilian Immunization Society, in a statement. Espirito Santo. “Even the strategy of vaccinating pregnant women is old, we do it for other diseases, like flu, whooping cough, tetanus and hepatitis B.”
Source: Terra
Ben Stock is a lifestyle journalist and author at Gossipify. He writes about topics such as health, wellness, travel, food and home decor. He provides practical advice and inspiration to improve well-being, keeps readers up to date with latest lifestyle news and trends, known for his engaging writing style, in-depth analysis and unique perspectives.





![A more beautiful life in advance: Vanessa and Silvi are ready to do everything to save sweat … [SPOILERS] A more beautiful life in advance: Vanessa and Silvi are ready to do everything to save sweat … [SPOILERS]](https://fr.web.img3.acsta.net/img/7f/38/7f38f0ec1b303b1d818aa5e477af477f.jpg)
