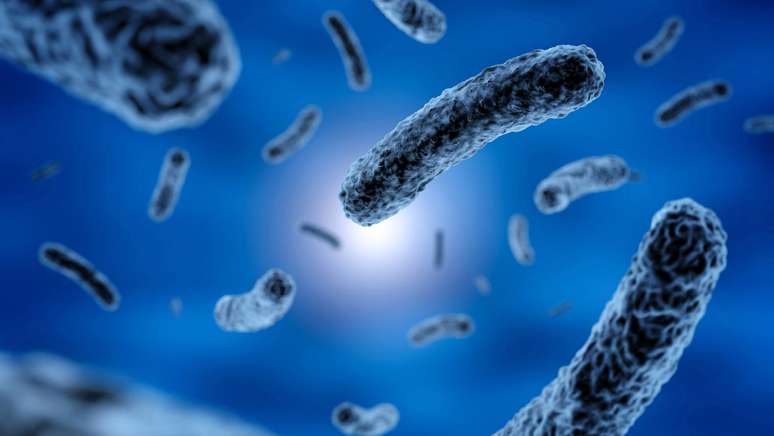
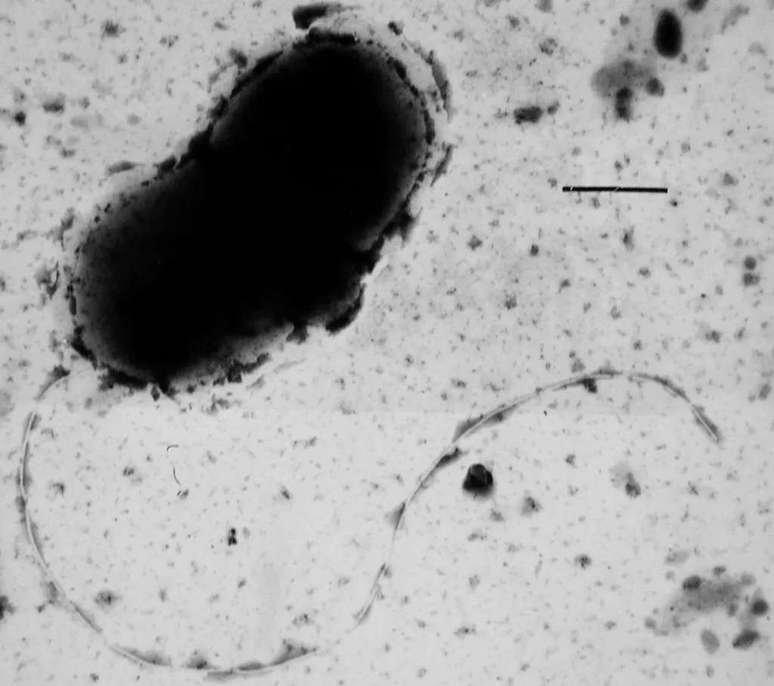
Scientists have discovered that a bacterium common in humid environments may be an important environmental driver of the accumulation of α-synuclein, linked to Parkinson’s disease.
A team of Finnish scientists has discovered that a microbe common to wet and marshy environments may play an important role in the development of Parkinson’s diseaseas it eliminates compounds that lead to the development of proteins in brain cells responsible for the formation of toxic clusters, linked to neurodegenerative diseases.
- Artificial intelligence can detect Parkinson’s through breathing
- Study uses smartwatch and cell phone to understand Parkinson’s disease
The research comes on the heels of previous studies, which have already demonstrated a connection between the presence of high concentrations of the strain’s bacteria Desulfovibrio in the stools of patients and increased severity in their cases of Parkinsons. With the discovery, diagnosing the disease may be easier in the future, and may also have pointed to a door to possible treatments targeting the bacterium and its excreted substances.
Parkinson’s past and present
Since James Parkinson first described the neurological disease some 200 years ago, much effort has been made to explain the causes of the marked loss of fine motor coordination with age. We’ve already found that small inclusions called Lewy bodies appear in cells in certain regions of the brain in patients with the condition, for example.
More recent research has found that most Lewy bodies are composed of the protein α-synuclein, which plays an important role in the release of neurotransmitters. It’s not yet clear how these lumps contribute to Parkinson’s disease, but concentrations of the protein, called protofibrils, are suspected to prevent nerve cells from functioning satisfactorily.
What is not yet known is the initial cause of α-synuclein aggregation and the answer is not exactly genetic: only 10-15% of cases are hereditary. Environmental conditions, then, are the main suspects, and there are already studies linking intestinal bacteria to the possibility of developing Parkinson’s symptoms. Now, the finger has been pointed at the main probable cause of the disease: the Desulfovibrio.
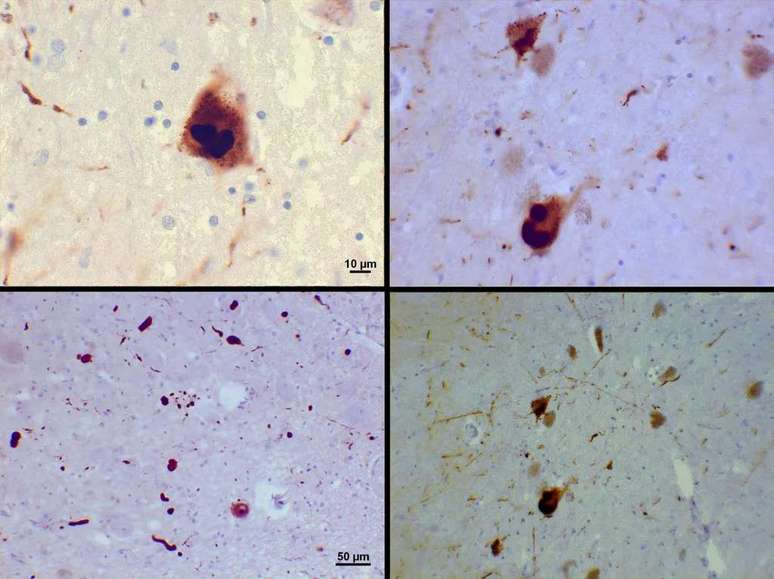
Reveal the bacteria
Per Saris, a microbiologist at the University of Helsinki, assembled a small team and collected fecal samples from 10 patients from Parkinsons and their healthy partners by isolating strains of Desulfovibrio present in their gut to further investigate the matter. Two control groups of different bacteria from a different kind of biology received samples of the nematode (a type of worm) Caenorhabditis elegans modified to express human α-synuclein.
Statistical analyzes carried out on microscopic observations of nematode heads showed that those receiving Desulfovibrio actually had a greater chance of producing α-synuclein clusters, as well as being larger. Desulfovibrio strains from Parkinson’s patients were better at aggregating proteins in roundworms than their healthy counterparts.
Worms with strains of patients with the disease still died in higher numbers than those in control groups. While we are very different from tiny creatures, the experiment already offers many opportunities for evaluation disease identification strategies and possible cures, even if we can’t do the same experiments with humans.
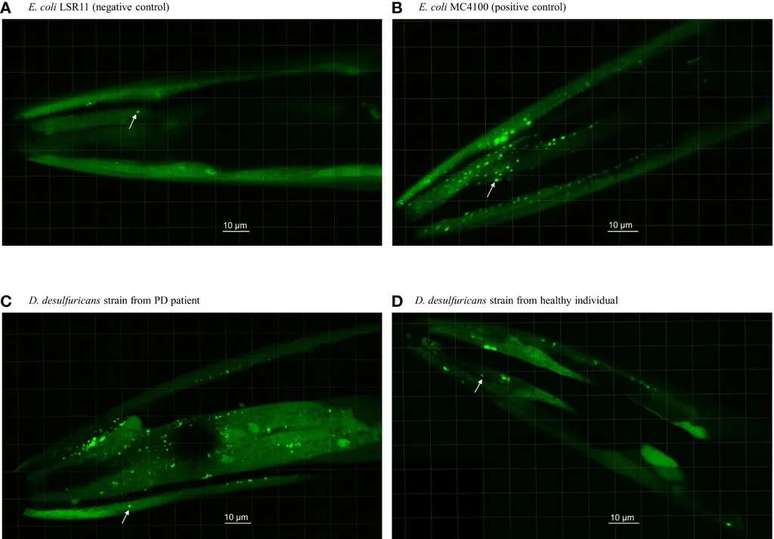
For the future, it is expected to control the progression of the disease using therapies that target the digestive system and nearby nerves rather than targeting the brain alone. After being eliminated from the intestines, the bacteria Desulfovibrio they stop forming α-synuclein clumps in organ cells, from where they travel to the brain via the vagus nerve, like prion proteins. Time will tell how we can take full advantage of the discovery.
Source: Frontiers of cellular microbiology and infections
Trending on Canaltech:
- 7 kids who made horror movies and how they are today
- Why do hospitals give gelatin to patients?
- Stephen Hawking predicted AI’s extermination of humanity almost a decade ago
- 5 reasons NOT to buy the Chevrolet Tracker Midnight
- Does Guardians of the Galaxy 3 have a post-credits scene?
- Oppenheimer | Robert Downey Jr looks unrecognizable in new trailer
Source: Terra
Rose James is a Gossipify movie and series reviewer known for her in-depth analysis and unique perspective on the latest releases. With a background in film studies, she provides engaging and informative reviews, and keeps readers up to date with industry trends and emerging talents.