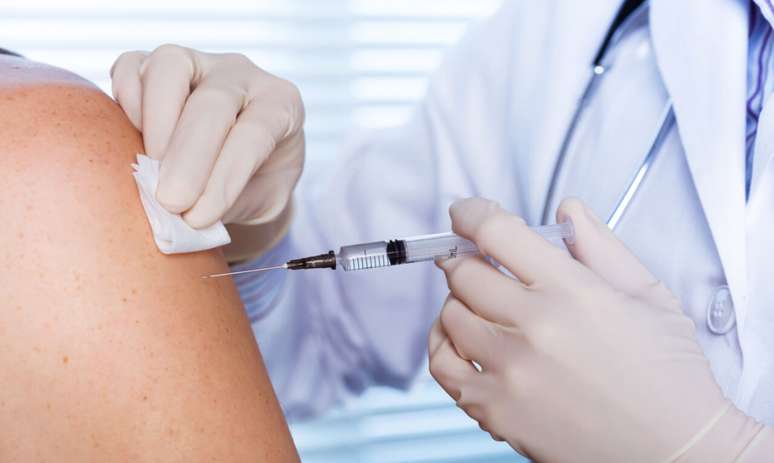
Find out how the new dengue vaccine approved by Anvisa in March was born, what it costs and for whom it is recommended
The Brazilian Association of Vaccination Clinics (ABCVAC) has announced the arrival of a new dengue vaccine in Brazil. The vaccine should be in the country next week. Qdenga, from Takeda Pharma Ltda., is made up of four different serotypes of the disease-causing virus, and was approved by the National Health Surveillance Agency (Anvisa) in March. According to the regulatory body, the dose provides broad protection against dengue.
html[data-range=”xlarge”] figure image img.img-6d11e321b084580dd0208df7c71d4fff8r4dx0ex { width: 774px; height: 463px; }HTML[data-range=”large”] figure image img.img-6d11e321b084580dd0208df7c71d4fff8r4dx0ex { width: 548px; height: 328px; }HTML[data-range=”small”] figure image img.img-6d11e321b084580dd0208df7c71d4fff8r4dx0ex, html[data-range=”medium”] figure figure img.img-6d11e321b084580dd0208df7c71d4fff8r4dx0ex { width: 564px; height: 337px; }
cost of immunization
In a note, ABCVAC reported that the price of the vaccine, initially available only in private laboratories, is expected to vary between R$ 350 and R$ 500 for the final consumer – that is, depending on the state. In São Paulo, for example, the maximum consumer price (PMC) authorized by Anvisa for clinics is R$ 379.40.
Who can get vaccinated?
According to Anvisa, children over 4 years of age, adolescents and adults up to 60 years of age can get the vaccine. With this, Qdenga becomes the first dose approved in Brazil for a wider audience. This is because the previously approved vaccine, Dengvaxia, was only indicated for those who already had dengue.
How many doses are needed and how effective is the vaccine?
The new vaccine will be available for subcutaneous administration in a two-dose schedule, with a three-month interval between applications.
The efficacy against dengue for all serotypes combined among seronegative individuals (with no prior dengue infection) was 66.2%. For HIV-positive individuals (who had a previous infection with the virus), the rate was 76.1%.
“The demonstration of Qdenga’s efficacy is primarily supported by the results of a large-scale, Phase 3, randomized, placebo-controlled study conducted in dengue-endemic countries to evaluate the efficacy, safety, and immunogenicity of the vaccine” , informed Anvisa.
Source: Terra
Ben Stock is a lifestyle journalist and author at Gossipify. He writes about topics such as health, wellness, travel, food and home decor. He provides practical advice and inspiration to improve well-being, keeps readers up to date with latest lifestyle news and trends, known for his engaging writing style, in-depth analysis and unique perspectives.





![A Better Life Preview: What’s in store for Tuesday, October 21, 2025 Episode 446 [SPOILERS] A Better Life Preview: What’s in store for Tuesday, October 21, 2025 Episode 446 [SPOILERS]](https://fr.web.img6.acsta.net/img/fe/c7/fec79870332faf9ba9cf244248ec57c8.jpg)


