Hospitalization cases with symptoms similar to botulism have generated alert in the country
Summary
Four people have been hospitalized due to complications caused by the use of counterfeit botulinum toxin. The CDC is ready to notify health care providers in several US states, and cases have been confirmed in Kentucky.
North American site New York Post reported that four people ended up in hospital due to complications caused by the application of counterfeit botulinum toxin (botox). According to the publication, the Centers for Disease Control and Prevention (CDC) is poised to issue a warning to healthcare professionals about “botulism-like illnesses.”
The website reported that people in several states across the country – including Illinois, Colorado, Kentucky, Washington and Tennessee – have become ill after receiving counterfeit Botox injections. Two cases of hospitalization occurred in Illinois and Tennessee.
NBC News also reported that four more crashes were confirmed in Kentucky on Thursday, the 11th. “The events led to a “multi-state outbreak investigation,” the CDC said.
Botulism is a rare poisoning caused by toxins produced by the bacterium Clostridium botulinum. This bacterium can be found in the soil, in poorly preserved or contaminated foods, and can also grow in anaerobic conditions, i.e. in the absence of oxygen.
When a person eats food contaminated with botulism toxin, they may develop symptoms such as muscle weakness, blurred vision, difficulty swallowing and breathing, as well as other serious neurological symptoms. If not treated quickly, botulism poisoning can lead to muscle paralysis and even death.
Anvisa has already warned
In 2023, the National Health Surveillance Agency (Anvisa) has issued an alert for healthcare workers and the population, after identifying cases of Botox counterfeiting.
The company that holds the registration of the Botox drug, Allergan Produtos Farmacêuticos Ltda., has communicated to Anvisa the identification in Brazil of two units of the counterfeit product with validity until April 2025.
The main differences between the counterfeit product and the original product lie in the labeling, information leaflet and packaging. The original medicine has a seal on the secondary packaging, which is not present on the counterfeit product.
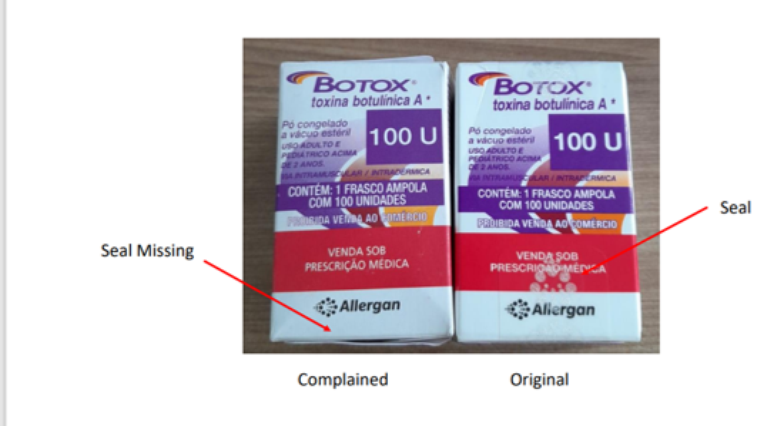

“Regarding this medicine, a preventive measure has been published in the Official Journal of the Union, through resolution 352 of 1 February 2023, which determines the seizure and prohibition of marketing, distribution and use of the counterfeit product”, informed the ‘Anvisa.
How to prevent
In an interview with Earththe dentist Mariana Oliveira, underlined the importance of knowing that only professionals with degrees in Biomedicine, Biological Sciences, Nursing, Pharmacy, Medicine and Dentistry have the authorization of their councils to carry out the application of botox.
Elizabeth Hale, associate clinical professor of dermatology at NYU Langone, also recommended getting treatment “in the hands of a board-certified dermatologist or plastic surgeon.”
“Often people are trying to save a little money. And it’s not the best idea if you don’t know exactly who is injecting you and what they’re injecting you with,” she said in an interview with the New York Post.
-1jy28ccc3rwoi.png)
Blood on the face and collagen: what celebrities do for their beauty
Source: Terra
Ben Stock is a lifestyle journalist and author at Gossipify. He writes about topics such as health, wellness, travel, food and home decor. He provides practical advice and inspiration to improve well-being, keeps readers up to date with latest lifestyle news and trends, known for his engaging writing style, in-depth analysis and unique perspectives.