Consequently, the FDA recommended the removal of the voluntary suspension from the use of elessys
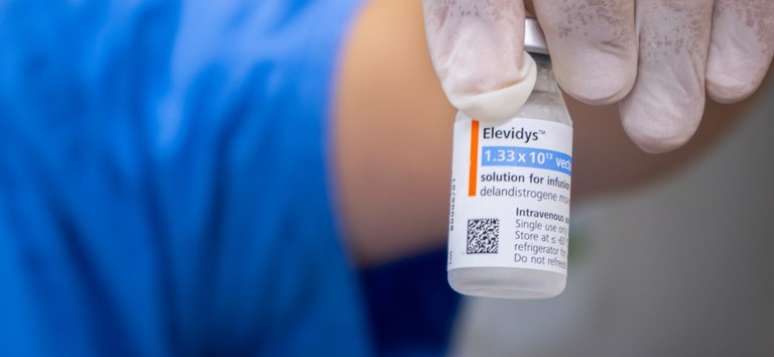
Food and Drug Administration (FDA), the United States regulatory agency, said that the death of an 8 -year -old boy with Duchenne muscle dystrophy (DMD) is not related to the use of Elessys. The remedy is the only gene therapy that can slow down the effects of the rare and serious condition, characterized by progressive muscle weakness and the degeneration of muscle tissue.
Receive the main news directly on WhatsApp! Sign up for the Earth channel
The child died in Brazil on June 7th, shortly after receiving the drug.
The opinion of the FDA confirms the declaration of the National Health Surveillance Agency (Anvisa), which relates death to a “serious viral infection intensified by immunosuppression”. Fatality, according to the Brazilian agency, presented an “improbable causal bond” with the drug.
With the results of the investigations, the US agency recommended the removal of the voluntary suspension of the drug, which costs about $ 17 million, one of the most expensive in the world. In Brazil, as a precaution, Anvisa had also temporarily suspended marketing, distribution, production, import, advertising and use of elessyidys.
“The decision was made scientifically prudent and preventive, given the emergence of new international data indicating a serious hepatotoxicity with a fatal outcome,” explained the agency in a declaration published on 25.
The recording of the drug in the country was granted to the Roche Farvil Brasil company in December 2024, exceptionally, only for pediatric patients with (from 4 to 7 years).
In a declaration, the pharmaceutical company has declared that, in Brazil, “follows the dialogue with Anvisa and other competent authorities to ensure that all decisions are aligned with the patient’s security and regulatory guidelines”.
According to Anvisa, quoting information from Roche, about ten Brazilians have already been treated with the drug. And until July 25, the Agency had received only two adverse notifications in addition to the aforementioned death. These two other cases were classified as “probable, with favorable and compatible evolution with the effects already described in the flyer and observed on clinical studies”.
“The elessidys flyer recommends postponing administration in case of active infection and supports vaccination as a preventive mechanism,” said Anvisa.
What is Duchenne’s muscle dystrophy?
Duchenne muscle dystrophy (DMD) is a genetic disease, characterized by progressive degeneration and weakness of the muscles, which control movements.
It is considered a rare disease: according to the Duchenne movement, it affects about 1 out of 5,000 people, which represent about 20,000 new cases per year. In Brazil, statistics are at least 300 new patients per year.
The boys are the main affected. The girls, in turn, can transport the defective gene, but have no symptoms in most cases.
Source: Terra
Ben Stock is a lifestyle journalist and author at Gossipify. He writes about topics such as health, wellness, travel, food and home decor. He provides practical advice and inspiration to improve well-being, keeps readers up to date with latest lifestyle news and trends, known for his engaging writing style, in-depth analysis and unique perspectives.