The expedition begins the analysis of Cabotegravir Rite of the Ministry of Health
Summary
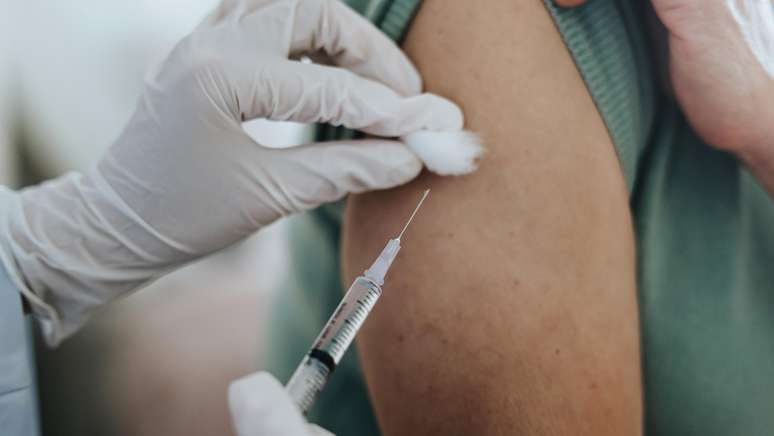
The pharmaceutical company asked Conitec to include injectable Cabotegravir, which prevents HIV up; The analysis of the Ministry of Health does not yet have a deadline.
The National Commission for the incorporation of technologies in the Unified Health System (CONITEC) will evaluate the inclusion of the injectable version of Cabotegravir, a drug indicated for the prevention of human immunodeficiency viruses (HIV) -1, in the public network.
Receive the main news directly on WhatsApp! Sign up for the Earth channel
Public services offer drugs, also known as pre-exposition prophylaxis (prep), in the form of tablets. However, it is necessary to take daily, the injectable can be applied every two months.
The request was submitted by the pharmacist GSK/VIIV Healthcare, with the support of the Brazilian Society of Infectology (SBI), the 12. The next step is the analysis of safety, effectiveness and cost-effectiveness of the treatment by Conitec. After the conclusion, those who decide the inclusion is the Ministry of Health. EarthThe company said there are no forecasts consultation or incorporation decision.
How does medicine work?
According to the National Health Surveillance Agency (Anvisa), Cabotegravir is the active ingredient of vocabb and an antiretroviral drug of the HIV incapacity class. It acts by binding to enzymatic integral and consequently blocking an essential step for the replication of the virus.
It was recorded in 2023 by Anvisa and launched in August 2025 in the private market.
Source: Terra
Ben Stock is a lifestyle journalist and author at Gossipify. He writes about topics such as health, wellness, travel, food and home decor. He provides practical advice and inspiration to improve well-being, keeps readers up to date with latest lifestyle news and trends, known for his engaging writing style, in-depth analysis and unique perspectives.


-1iyq160aoa4ag.png)
![Everything starts here: What awaits you on Thursday of September 18, 2025 in the 1266 episode [SPOILERS] Everything starts here: What awaits you on Thursday of September 18, 2025 in the 1266 episode [SPOILERS]](https://fr.web.img3.acsta.net/img/44/34/4434fec4599e79e4c76ca28c3f25362a.jpg)



