Agency directors have authorized the use of the booster immunization in the population over the age of 12; Pfizer says the doses should arrive in the country in the coming weeks
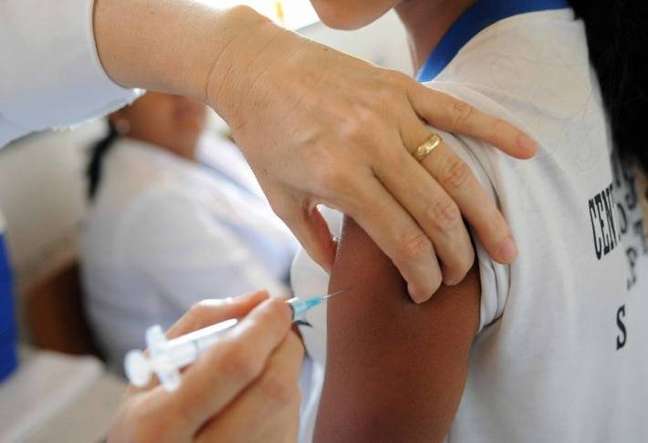
BRASILIA – A National Health Surveillance Agency (Anvisa) approved, this Tuesday 22, the emergency use of Pfizer’s bivalent vaccine against COVID-19🇧🇷 Anvisa’s decision, unanimously approved, allows the application of the immunizer as a booster dose in the population over the age of 12. At the same meeting, the directors of the agency established that the use of masks at airports and on flights in the country will become mandatory. This measure will come into force on the 25th.
According to the agency, in-flight service will not be banned and passengers will be able to remove their masks to eat or even drink liquids at other times during the flight. The obligation applies to all Brazilian airports and also to domestic flights. In the case of international travel, the rule only applies to boarding in Brazil and disembarking from an international flight in Brazilian territory.
The theme of the mask was included on the agenda for discussion by the Anvisa board at the beginning of the meeting. Director Daniel Pereira, rapporteur for the process, voted to strengthen the recommendation for the equipment and not revert to the requirement to use it. But Pereira ended up defeated by the other four advisers.
The dissent vote was presented by director Alex Campos Machado, who said that despite the progress of vaccination, the change in the covid scenario “alerts the whole world”. Barra Torres highlighted, at the beginning of the session, that the coronavirus “is still a great concern for everyone in the world, given the condition deriving from the emergence of variants”.
“We are still observing the growth of contagion rates, hospitalization rates and also mortality rates on the national territory. Evidently not in a number similar to the years we have, but still a very worrying number,” he said.
The adoption of non-pharmacological measures, such as the use of the mask, was addressed by Anvisa in a meeting with epidemiologists, health specialists and representatives of the National Council of Health Secretariats (Conass) and the National Council of Municipal Health Secretariats ( Conasems). The meeting on the covid scenario took place virtually this Monday 21.
Anvisa had made the wearing of masks mandatory at airports in December 2020, at the height of one of the covid surges in the country. The agency eased the measure in August this year, about a year and eight months after the charge began. Three months ago, protective equipment became “a recommendation, especially for people with flu symptoms and the most vulnerable public, such as the immunocompromised, pregnant women and the elderly”.
Vaccine
In a note, Pfizer reported that it estimates that the arrival of bivalent vaccines in Brazil will take place “in the coming weeks”. According to the pharmaceutical company, “Pfizer’s current contract to supply vaccines to the country includes the delivery of potential vaccines adapted to new variants and/or for different age groups”.
“We emphasize that the original monovalent vaccine is still available for immediate use in health centers and continues to be an important tool in the fight against covid-19, both as a primary schedule and as a booster dose,” said the agency.
The new immunizers will have a gray lid, different from the monovalent ones, to help differentiate the vaccines. The new versions do not require dilution for application.
The bivalent vaccines they are more up to date and contain a mixture of SarsCov-2 virus strains. In this way, immunizers can guarantee greater protection against the new variants. Brazil is experiencing a new wave of covid-19, which has experts worried due to increased resistance to vaccine barriers and the transmissibility of new strains of the coronavirus.
Director Meiruze Sousa Freitas, rapporteur of the Anvisa trial, voted for the approval of the new vaccines. He stated that subvariants BA4, BA5, BQ1.1 are currently the majority of new covid cases. He also pointed out that there is an increase in cases and hospitalizations, especially in groups that have not received full vaccination.
“The pattern of deaths that Brazil is still facing is not natural and therefore, I feel that preventive measures, for example wearing masks, hand hygiene remain an important part of our response to covid-19,” said the speaker.
Meiruze Freitas also advised the population to continue taking booster doses at the time indicated with monovalent vaccines. “Individuals eligible for a booster dose, especially those in groups at highest risk of developing severe COVID-19, should not delay planned vaccination to wait for access to the bivalent vaccine.” she said. “All of the approved booster vaccines help enhance the protection gained from previous doses of the vaccine and help provide protection against serious illness and death from COVID-19.”
Today, the subvariants that most concern health authorities around the world are BQ.1 and XBB, both of the Ômicron lineage, a variant against which Pfizer’s bivalent vaccine has specific protection, unlike the immunizers available in Brazil.
According to experts, the immunizers available today in health centers around the country provide minimal protection against the new variants, but people must take as many booster doses as needed. For immunosuppressed peoplelike recent transplant patients, people with cancer or lupus, infectious disease specialists say immunization with the most up-to-date bivalent vaccine is very important.
Pfizer has filed two temporary emergency use authorization applications for emergency use of the vaccines. The first request, referring to the bivalent with the Ômicron BA1 subvariant, was filed on August 19, 2022. The second, referring to the version containing the BA.4/BA.5 subvariant, was filed on September 30, 2022.
Since then, the agency has been analyzing the question. Before the meeting was convened, the agency was silent about the deadline for completing the procedure. Anvisa and the Ministry of Health also said they had no information indicating when the new doses could start to be applied.
The urgent use authorization is governed by an agency resolution. After receiving the request, the Agency has 30 days to complete the evaluation. The deadline is interrupted if it is necessary to request the company to integrate information or clarify the quality, efficacy and safety data presented.
Delay
The Transition Group responsible for health claims confirmed this 85 million Brazilians have not yet taken the third dose against covid-19. Members are scheduled to meet with the Minister of Health, Marcello Queirogathis Wednesday the 23rd.
“It is a situation of vulnerability, there are no doses for children aged 3 and over, the booster dose for children over 10 has not been given. It is a situation that cannot be called normal. We may be much better prepared to receive this new wave (from covid), to face this new pandemic wave that is already present in the country”, said Arthur Chioro, a health doctor who makes up the health group.
Until December 31, the doctor recalled, the responsibility for purchasing vaccines and fulfilling contracts rests with the current leadership of Jair Bolsonaro. A meeting with specialists is also scheduled for Thursday 24: “Thursday morning we are calling more than 30 scientific societies to dialogue with us on this very issue”. The technical table has already requested the data from the Ministry of Health and is awaiting information.
The report asked the Ministry of Health if there is already an agreement with Pfizer for the acquisition of bivalent vaccines and the number of doses that the folder will purchase. There was no return.
The federal government has sent next year’s budget proposal to Congress, with a forecast of BRL 8.655 billion for the acquisition and distribution of immunobiologics for disease prevention and control, which includes vaccines against covid-19 . The value is 507 million reais less than that sent, in 2021, to this year’s budget law.
🇧🇷The best content in your email for free. Choose your favorite Terra newsletter. Click here!
Source: Terra
Ben Stock is a lifestyle journalist and author at Gossipify. He writes about topics such as health, wellness, travel, food and home decor. He provides practical advice and inspiration to improve well-being, keeps readers up to date with latest lifestyle news and trends, known for his engaging writing style, in-depth analysis and unique perspectives.




![A more beautiful life in advance: Apollin between torn love and friendship? … What are you waiting for from the week of 2025 to May 16, 2025 [SPOILERS] A more beautiful life in advance: Apollin between torn love and friendship? … What are you waiting for from the week of 2025 to May 16, 2025 [SPOILERS]](https://fr.web.img6.acsta.net/img/df/26/df261d50edf7203927ce00773c7af77a.jpg)

![Tomorrow belongs to us in advance: Alex is preparing to go? … What are you waiting for from the week of 2025 to May 16, 2025 [SPOILERS] Tomorrow belongs to us in advance: Alex is preparing to go? … What are you waiting for from the week of 2025 to May 16, 2025 [SPOILERS]](https://fr.web.img5.acsta.net/img/8e/b2/8eb2158bd34199ce9704a989e16e38d9.jpg)

