The US public health agency has identified 68 patients in 16 US states with a rare strain of the bacteria
html[data-range=”xlarge”] figure image img.img-c8437b80e79570af495a6e503221b625z4dalvvw { width: 774px; height: 435px; }HTML[data-range=”large”] figure image img.img-c8437b80e79570af495a6e503221b625z4dalvvw { width: 548px; height: 308px; }HTML[data-range=”small”] image figure img.img-c8437b80e79570af495a6e503221b625z4dalvvw, html[data-range=”medium”] figure image img.img-c8437b80e79570af495a6e503221b625z4dalvvw { width: 564px; height: 317px; }
US health officials say the eye drops may have killed one person and left several other serious injuries due to drug-resistant bacterial contamination.
The Centers for Disease Control and Prevention (the US public health agency CDC) have identified 68 patients in 16 US states with a rare strain of Pseudomonas aeruginosa.
The strain had never been found in the United States prior to this latest outbreak.
In addition to one death, eight patients suffered vision loss and four had to have their eyes surgically removed.
Most patients diagnosed with the infection reported using eye drops and artificial tears, according to the CDC.
Ten different brands have been identified as possibly linked to the outbreak, the CDC said. Eye drops manufactured in India and imported into the US under two brand names were pulled from shelves in January and February.
In January, the CDC warned people to stop using EzriCare and Delsam Pharma artificial tears. The following month, the company that owns the brands, Global Pharma, carried out a voluntary recall following a recommendation from the Food and Drug Administration (FDA), the health agency of the United States government.
The vials opened by the patients were tested. The CDC detected the bacteria in the samples. Unopened vials are examined in the laboratory to determine if contamination has occurred during the manufacturing process.
Last week, a Florida woman sued the drug company, claiming an infection she suffered after using the product forced doctors to remove one of her eyes.
A lawyer for the woman attributed the contamination to the lack of preservatives in the eye drops.
“There are probably a lot more people who have had infections that I don’t know about,” attorney Natasha Cortes told NBC News.
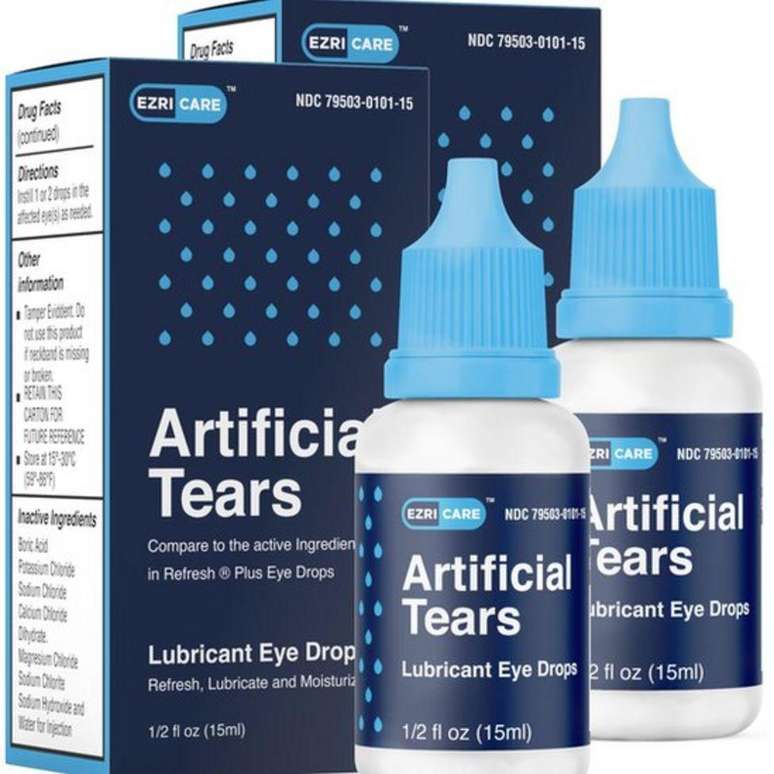
A representative for EzriCare said tests thus far have not conclusively shown a link between the outbreak and its products.
“As far as possible, we are contacting customers to advise against continued use of the product,” a spokesperson said.
“We also immediately contacted the CDC and the FDA and indicated our willingness to cooperate with any requests they make of us.”
The CDC said anyone who has used the recalled products and is now experiencing symptoms should contact a doctor.
Symptoms include yellow, green, or clear eye discharge, discomfort or pain, redness, blurred vision, and increased sensitivity to light.
Last week, the FDA issued separate recall notices for some eye drop products distributed by Pharmedica and Apotex after the companies said they voluntarily pulled them from shelves.
Eye drops and eye washes were used by an estimated 117 million Americans in 2020, according to Statista, a market research firm.
Source: Terra
Ben Stock is a lifestyle journalist and author at Gossipify. He writes about topics such as health, wellness, travel, food and home decor. He provides practical advice and inspiration to improve well-being, keeps readers up to date with latest lifestyle news and trends, known for his engaging writing style, in-depth analysis and unique perspectives.