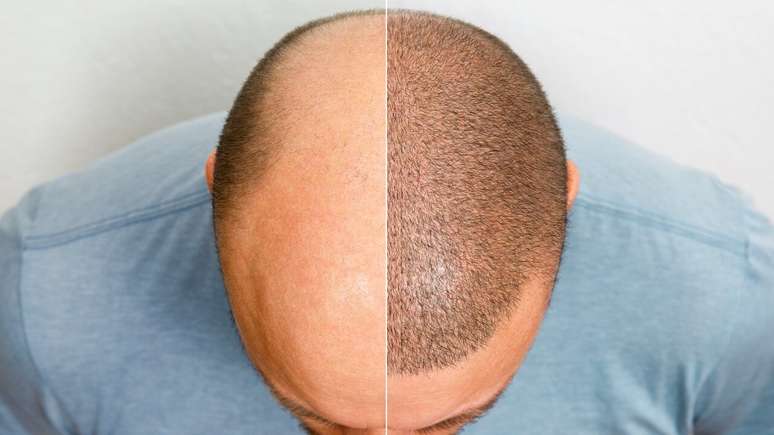
The drug has received agency approval for the treatment of hair loss
A Anvisa (National Health Surveillance Agency) released the medicine in October 2023 Olumiantbetter known as Baricitinibfor treatment against hair loss.
In this way, this drug may be useful to adults at risk of alopecia areataa disease responsible for this hair loss.
Please note that this is a new indication, i.e. Olumiant Baricitinib has already been approved for procedures against Covid-19, dermatitis and arthritis and must also be prescribed to people over the age of 18. Its cost is, on average, R$ 4,000.00.
Action of Olumiant Baricitinib and what is alopecia
The SBD (Brazilian Society of Dermatology) defines alopecia as a significant loss of hair or hair and is also given several names in different examples, which include areata, traumatic, androgenetic, effluvial and seborrheic.
This type of disease does not “select” a public, that is, it affects people of both sexes. Its onset is due to the inflammatory process, which begins at the root of the hair.
Other theses also underline that alopecia areata is influenced by emotional problems, infectious problems, type 1 diabetes and genetic predisposition. However, it does not qualify as a contagious condition.
Source: Terra
Ben Stock is a lifestyle journalist and author at Gossipify. He writes about topics such as health, wellness, travel, food and home decor. He provides practical advice and inspiration to improve well-being, keeps readers up to date with latest lifestyle news and trends, known for his engaging writing style, in-depth analysis and unique perspectives.