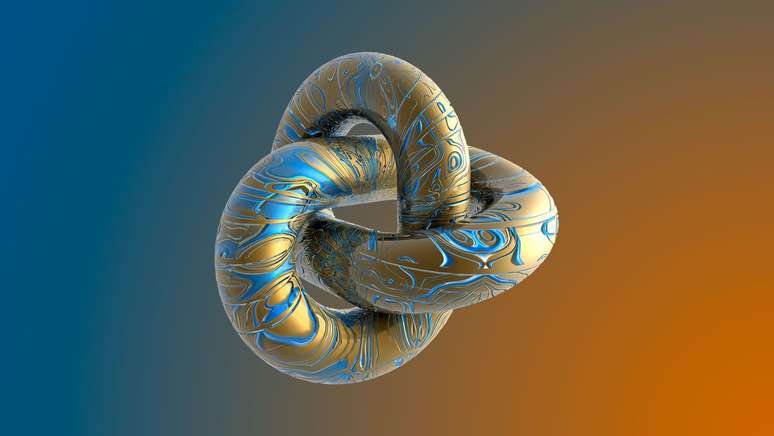
Surpassing the previous record, broken in 2020, chemists have tied the tightest knot in the world, reaching the theorized limit of bonding by atomic particles
Completely by chance, the chemists tied the smallest and tightest knot ever, surpassing the previous record holder record book – the incredible feat is made up of just 54 atoms, which come together three times to form a cloverleaf knot. The shape, which resembles a clover, has no free ends, and is the simplest way to form a trivial knot, a fundamental element of the mathematical theory of knots.
- DeepMind’s artificial intelligence solves the problem that has plagued mathematicians for more than 40 years
- Scientists find new structures in DNA molecules beyond the double helix
Four years ago, in 2020, Chinese chemists crossed a chain of 69 atoms three times to create the same shape, but researchers from the University of Western Ontario, Canada, and the Chinese Academy of Sciences teamed up to beat the record. As the ratio of atoms to crossovers decreases, the strength of the molecular knot increases. The 2020 node had a crossover ratio (RC) of 23 and the most recent one had 18.
Molecular knots and science
Most organic molecular knots have an RC between 27 and 33, but experts don’t know how small or tight they can continue to make them. Some quantum chemical calculations suggest that the most stable cloverleaf structure would be about 50 molecules long, meaning we’re getting very close to the theoretical limit.
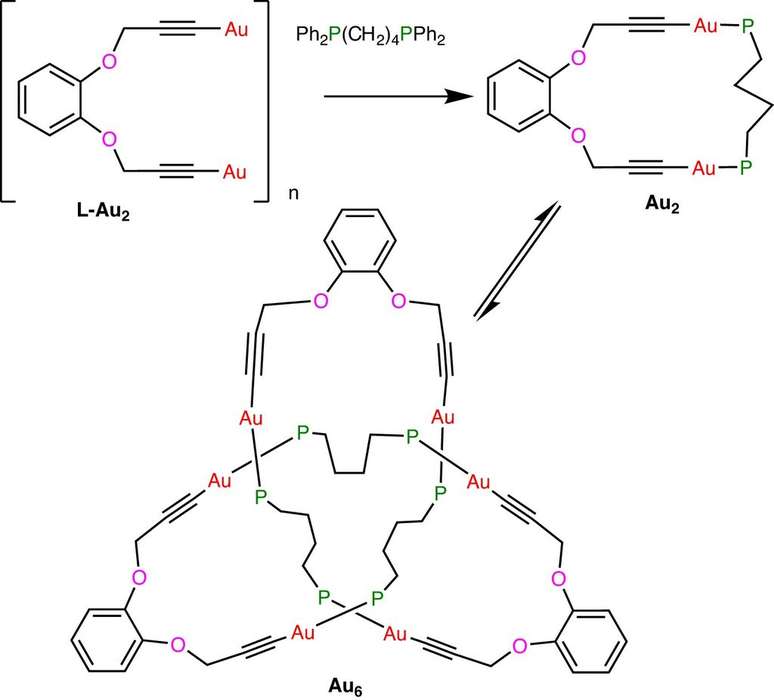
With this feat, scientists are closer than ever to the microscopic knots that form naturally in the DNA, RNA and many other proteins in our body. According to the researchers, plastics and polymers can also be produced in more efficient ways: their properties depend on the size of the knotted structure.
To the website News scientist, the chemist Richard Puddephatt told how the event happened, by chance. He and his colleagues worked with metal acetylides in the lab – they are alkynes, a type of hydrocarbon – with the hydrogen removed from the tip.
The product is very useful in chemical reactions carried out by scientists. When they were linking the gold acetylide with another carbon structure called a diphosphine ligand, the team ended up creating a cloverleaf knot instead of a gold chain (or catenane). Since 1989, chemists have attempted to create molecular knots by guiding helical chains into desired structures via metal ions.
The 2020 knot was made by bending and twisting the molecular chain, and when the metal atoms are removed at the end of the process, the knot can no longer be untied. The new golden knot is different, as it curls itself. It’s a complicated system, and scientists admit they don’t know how it works, but they say they’re excited to create similar systems in the future.
Source: Nature, Nature communications with information from News scientist
Trends on Canaltech:
- Brazil x Ecuador | Where to watch the national team match at the Pre-Olympics
- Windows 3.11 is still used in German railway operations
- The Stanley bottle and cup contain lead, but are safe
- NASA space probe takes a photo of the Moon and shows the Japanese SLIM lander
- Call of Duty | The video with the canceled version of the game shows the missions in space
- The alleged Snapdragon 8 Gen 4 shows insane performance during the test
Source: Terra
Rose James is a Gossipify movie and series reviewer known for her in-depth analysis and unique perspective on the latest releases. With a background in film studies, she provides engaging and informative reviews, and keeps readers up to date with industry trends and emerging talents.